Medicines and medical devices regulation – guidance from 1 January 2021
PAGB has produced detailed guidance for members on what you need to know to prepare for the end of the transition period. Resources include a detailed summary of MHRA ‘standstill’ guidance on regulating medicines and medical devices from 1 January 2021.
(member log-in required). Not a member? Find out more about PAGB membership.
In the period after the UK’s withdrawal from the EU and the subsequent transition period to the end of 2020, there should be no fewer consumer healthcare products available in the UK and those products should be no less safe.
PAGB priorities for a future EU/UK trade deal are:
- To ensure patient safety, to avoid duplicating processes and to minimise supply chain disruption, the UK and EU should agree appropriate Mutual Recognition Agreements for medicines, medical devices and food supplements standards, including the recognition of good manufacturing practice (GMP).
- The UK and the EU should aim for zero tariffs and free-flowing goods on all consumer healthcare products, ingredients and components
In addition, PAGB sees theoretical opportunities arising from divergence from the EU on some aspects of medical device and food supplements regulation and is currently exploring these opportunities in its working groups.
EU Exit and the consumer healthcare industry
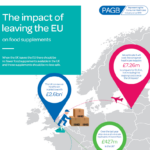
Over-the-counter (OTC) and self care products are medicines, medical devices and food supplements that can be bought from a pharmacy or other retail outlet without a prescription. PAGB members make well known and trusted products that people in the UK use every day to maintain good health and treat self-treatable conditions. The UK market for OTC and self care products is worth £2.73bn a year (Nielsen Scantrack, data to WE 28.12.19).
Over-the-counter medicines, self care medical devices and food supplements are rightly highly regulated products. This is imperative to protect public health. Regulation of these products in the UK must continue to be sensible, effective and proportionate, without becoming overly burdensome.
PAGB and its member companies are committed to working with Government departments and agencies, including MHRA, to ensure negotiations over the terms of the UK’s future trading relationships take the needs and priorities of the consumer healthcare industry into account.
PAGB – working on behalf of the consumer healthcare industry
The triggering of Article 50 on 29 March 2017 marked the start of over two years of negotiations between the UK and the EU in preparation for the UK’s exit from the European Union. PAGB has been working with our members and with Government and regulators as well as organisations in the health, pharmacy, pharmaceutical, food and medical devices sectors following the UK’s exit from the European Union and will continue to do so as the UK and EU negotiate the terms of the future economic partnership.
Our work in this area is focussed on the practical considerations relating to transitional arrangements as the industry adapts to new processes and requirements. In particular, our members’ priorities are to ensure that there continues to be an effective regulatory system and a clear and smooth transition to the new arrangements. As an industry, we also have an interest in the UK negotiating position on regulatory and supply chain issues, trade and the movement of people as it applies to working arrangements in the UK and Europe.
Northern Ireland Protocol: We are closely involved in ongoing discussions about the impact of the Northern Ireland Protocol. Our priority is a negotiated settlement supported by all parties. As the expert voice of the industry, we have shared our members’ views and concerns, including data on the potential effects of the Protocol on access to medicines, with Government and Parliamentarians. View our written submission and oral evidence to the House of Lords European Affairs Sub-Committee, which was also shared with Lord Frost in this letter from the Committee Chair, Lord Jay of Ewelme.
PAGB continues to work closely with our partners in the Association of the European Self Medication Industry (AESGP) to influence the direction of policy coming from the EU on over-the-counter medicines, self care medical devices and food supplements.
PAGB work on EU Exit is led by our Chief Executive with support from the wider team. Our member companies are involved in this process through their participation on special committees and working groups.
PAGB keeps members up to date on developments relating to EU Exit on a regular basis via information in the members area of our website, through our regulatory intelligence updates and via our EU Exit news blog and weekly EU Exit newsletter.
EU Exit publications and resources
PAGB has produced briefing papers, infographics and more to help explain the impact of the UK’s departure from the EU on access to OTC medicines, medical devices and food supplements. These resources are available to download for free. Please note, owing to the changing regulatory and policy landscape relating to EU Exit, these materials may have been or need to be updated.